Theme: Future Prospective and New Era Technologies in Biosimilars and Biologics
Biosimilars Congress 2021
Conference Series LLC Ltd, Scientific Event Organizer and the World Class Open Access Publisher invites all the participants from all over the world to attend “World Biosimilars & Biologics Congress” (Biosimilars Congress 2021) slated on November 26-27, 2021, Vancouver, Canada which covers on all aspects of the pharmaceutical sciences, manufacturing, quality with strong emphasis on originality and scientific quality. This Biosimilars Congress 2021 setting a platform for all the budding Scientists and Researchers to present their real-time work and share their views and aspects related to the theme of the Conference. The Organizing committee is gearing up for an exciting and informative Conference program including plenary Lectures, Symposia, Workshops on a variety of topics, Poster presentations and various programs for participants from all over the World.
Biosimilars Congress 2021 conveys recent developments in Pharma drug marketing and production of Pharma drugs and contract manufacturing. A complete knowledge of a scientific discipline that described the effects of Biosimilars drug marketing and Biosimilars Pharma now explores the Scope of Biosimilars drug marketing in industry & provides detailed market, technology, and industry analyses to help readers quantify and qualify the market for prescription generic drugs. Important trends are identified and sales forecasts by product categories and major country markets these are based on industry sources and considered assessment of the regulatory environment, healthcare policies, demographics, and other factors that directly affect the generic drug market. The wider economic environment is also considered.
Biosimilars:
A Biosimilars is a biologic restorative item profoundly like another previously affirmed natural prescription (the 'reference medication'). Biosimilars are endorsed by similar gauges of pharmaceutical quality, security and adequacy that apply to every single organic drug. Biosimilars are authoritatively affirmed forms of unique "pioneer" items and can be fabricated when the first item's patent expires. Reference to the trailblazer item is an indispensable part of the endorsement.
Biologics:
A Biologic medication (biologics) is an item that is created from living beings or contain segments of living creatures. Biologic medications incorporate a wide assortment of items got from human, creature, or microorganisms by utilizing biotechnology. Kinds of biologic medications incorporate immunizations, blood, blood segments, cells, allergens, qualities, tissues, and recombinant proteins. Biologic items may contain proteins that control the activity of different proteins and cell forms, qualities that control creation of imperative proteins, altered human hormones, or cells that produce substances that smother or initiate segments of the invulnerable framework. Biologic medications are some of the time alluded to as biologic reaction modifiers since they change the way of activity of regular biologic intracellular and cell activities.
Emerging Biosimilars in Therapeutics:
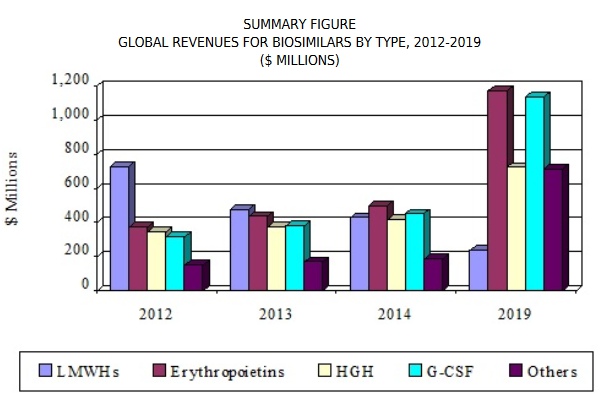
Biosimilars Market is experiencing a growth at an exponential rate. Presently around 700 biologics are making progress in the research pipelines of nearly 250 biopharma companies. Biosimilar insulins have already started revolutionizing the future drug development in the realm of diabetology. Biosimilars of Adalimumab, Etanercept, Rituximab, Peg-Filgrastim, Trastuzumab are expected to hit the market soon. Biosimilar of Humatrope, biosimilar of Eprex, biosimilar of Neupogen, biosimilar of Remicade have already been enjoying a greater market share in Europe than the reference product itself. The proportion of different biosimilars that reached market are Low Molecular Weight Heparins 44%, Epoetins 19%, HGH 11%, G-CSFs 7%, Interferons 6%, Insulins 5%, Others 8%.
Pharmacovigilance challenges in Biosimilars:
This session of the Biosimilars 2021 will investigate the future and FDA activities that have just been declared to incorporate upgraded following and follow-up of post promoting observation issues, arranged enhancements in AERS, and pilots of new post market drug-monitoring strategies and ADR related issues. Biosimilar rules for pharmacovigilance practice and pharmacoepidemiology are the focuses that will be laid emphasis in this session. U.S. normal yearly spending development from 2002 to 2007 was 16% for biologics, contrasted and 3.7% for drugs. In same extent pharmacovigilance for biosimilars has been nearly more than other pharmaceutical items.
Biological Medicine:
Biological Medicine works with the biology of the body and its natural healing capabilities as well as the spiritual, emotional and physical aspects of disease. Disease means that the body’s regulation is not working properly and needs to be brought back into its natural dynamic state where the immune system is in full regulation. It therefore looks for root causes for the presenting symptoms of disease the underlying factors causing a person to present with a certain illness. These root causes may consist of several factors which have built up over time and can include; diet, food allergies, intestinal disturbances, family history, stress, environmental factors, heavy metals, dental problems, hyperacidity, trauma, exposure to bacteria or viruses or electromagnetic disturbances.
Biopharmaceutical Informatics:
Biopharmaceutical informatics endeavors to use information technology, sequence-and structure-based bioinformatics analyses, molecular modelling and simulations, and statistical data analyse towards biologic drug development. Development of databases containing the experimental data on biophysical stability, safety along with molecular sequence
- Applications of computation in biologic drug development
- Physics-based molecular modelling
- Protein sequence-structural contexts and degradation reaction mechanisms
- De risk biopharmaceutical development
- Creation of databases and data mining
- Pre-clinical immunogenicity risk assessment of biotherapeutics
Chemical and Analytical Strategies for Biosimilars:
While small changes in these parameters can impact the various chemical permutations for a given biotherapeutic, innovators can, under certain circumstances, change the host cell, fermentation process, purification process, and even manufacturing site, but the product can be validated without undertaking a complete new product development review
- Biochemical and Biosimilar Characterization
- Biosimilars multimodal techniques
- Biosimilars bioanalytical methods
- Bioassay for comparability and potency testing
- Biosimilars LC/MS analysis for discovery, preclinical and clinical programs
- Biosimilars GMP protein analysis
- Biosimilars electrophoresis
Bioavailability and Bioequivalence:
Bioavailability is defined as amount of drug available to the target site after systemic circulation and Bioequivalence is the similarity of two drugs must release the active ingredient at the same amount, rate &quality. In this process the new drugs are tested for their stability studies. The new formulations undergo the analytical process to get approved by the FDA as a safe and effective dosage form. It is frequent to compare new drug with the standard product as a reference.
- In vivo & In vitro drug studies
- Accelerated stability studies
- Chromatography types and techniques
- HPLC to monitor β-lactam plasma
- Chemically, therapeutically and physically bioequivalent.
- Cationic prodrugs as dual gene reagents
Biosimilars Development in Markets:
A Biosimilars bio therapeutic item is comparative (but not identical) as far as quality, wellbeing, and viability to an effectively authorized reference item. Not at all like nonspecific little particles, it’s hard to institutionalize such naturally complex items in light of convoluted assembling forms. The worldwide biosimilars advertise is developing quickly as licenses on blockbuster biologic medications terminate and other medicinal services parts centre on lessening of expenses. Biologics are among the most elevated cost medicines on the worldwide market today, which suggests the requirement for minimal effort choices. In developing markets, biosimilars officially offer more moderate costs, which are alluring, as well as crucial to economies where costly medications are not monetarily achievable.
- Biosimilars China and Asia Pacific
- Biosimilars India
- Biosimilars USA
- Biosimilars Europe
Immunogenicity of Biosimilars:
Biologics and biosimilars are biotechnology-inferred proteins and can possibly be profoundly immunogenic. The results of a resistant response to a helpful protein go from transient responses to extreme hazardous conditions. Tolerant , infection and item related variables impact the advancement of immunogenicity, and appraisal of immunogenicity is constantly required for endorsement of a biosimilar. Appraisal of a biologic immunogenicity profile is directed in comparability clinical preliminaries to affirm that the immunogenicity profile of the biosimilar is like that of its reference biologic. An outline of clinical preliminaries evaluating the immunogenicity of infliximab biosimilars in patients with rheumatoid joint pain and ankylosing spondylitis and the outcomes from observational investigations in patients with gastroenterology maladies are displayed. Because of the little patient populaces examined in the comparability clinical preliminaries, the safe reaction may not be caught preceding permitting of the biosimilar. In this manner, continuous pharmacovigillance is required to guarantee the protected utilization of these novel treatments.
Clinical Development of Biosimilars:
This track remembers Clinical preliminaries for significant illnesses Risk the board, and quality issues, Case ponders, and clinical models, Transgenic creatures, Targeted cell line improvement, Clinical biosimilar tracks.docx PK/PD thinks about, Toxicological examinations and Aspects of genotoxicity tests. Clinical preliminaries are planned in stages I-IV in order to get a reasonable image of the medication up-and-comer in regard to its pharmacokinetics and pharmacodynamics parameters. Biologics additionally speak to more than 40 percent of the medications in each of the preclinical, Phase I, Phase II, and Phase III preliminary stages.
After the successful completion of the Biosimilars Congress 2021 conference series, we are pleased to welcome you to the "World Biosimilars & Biologics Congress" The congress is scheduled to take place on November 26-27, 2021 in the beautiful city of Vancouver, Canada. This Biosimilars Congress 2021 Conference will give you exemplary experience and great insights in the field of research.
As per market researchers the global "Biosimilars Market by Product (Recombinant Non-Glycosylated Proteins (Insulin, rHGH, Interferon), Glycosylated (mAb, EPO), Peptides (Glucagon, Calcitonin)), Manufacturing Type (In-house, Contract), Disease (Oncology, Autoimmune) - Global Forecast to 2023", The biosimilars market is expected to reach USD 23.63 Billion by 2023 from USD 5.95 Billion in 2018, at a CAGR of 31.7%. Granulocyte colony-stimulating factors (G-CSFs) reached $379.3 million in 2013. This segment is expected to increase from $453.6 million in 2014 to $1.1 billion by 2019, a CAGR of 20.2% from 2014 to 2019.
Importance & Scope:
The European-based pharmaceutical industry makes a major contribution to the Europe, not just in financial terms but also in terms of high-trait employment. Globally Pharma Market ranges from $870-$900 billion and in Europe $260-$280 billion.
A global biosimilars strategy:
Developed markets: Developed markets, with the exception of the United States, represent the greatest biosimilars presence today. Most biosimilars manufacturers have been and remain focused on the developed markets – whether it is for their historic and current opportunities (EU) or for their future market potential (United States, Japan). Dedicated regulatory pathways set the foundation for stringent, abbreviated approval processes which, in turn, have fed investor enthusiasm. Biosimilars adoption in developed markets has been primarily payer-driven, especially in European markets, given payers’ urgent, unmet need to contain public health care expenditures. Further market uptake has been slowed by prescribers’ skepticism and low patient awareness. Still, developed markets continue to have the highest number of biosimilars molecules in development – estimated at 29 in Europe, 19 in the United States and seven in Japan.
Emerging markets: In today’s emerging markets, biosimilars are still nascent, with little to no presence. However, in contrasting emerging markets with developed markets, the limited patient access to affordable biologics and the openness of physicians to low-cost therapies may offer potentially significant opportunities. Today, emerging markets represent a snippet of total world biologic sales in value, less than seven to eight percent (versus 48.6 percent in the United States).ix Treatment rates for flagship biologics are still low compared to developed markets, despite existing demand. For example, the treatment rate of Mab Thera® in Brazil is three times lower than in the UK and six times lower than in the US.x Additionally, a recent Kantor Health Survey found that 20 percent of emerging market autoimmune patients use a biologic, with the distribution of biologics varying from 29 percent in China to 12 percent in Russia and a mere 6 percent in Brazil.xi This may indicate the presence of large pockets of non-consumption, especially within the growing middle class.
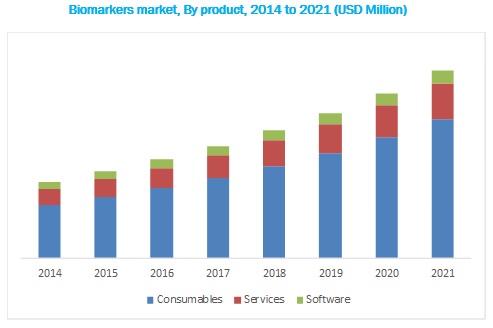
Biomarkers Market Analysis:
Based on product, the biomarkers market is segmented into consumables, services, and software. The consumables segment accounted for the largest share of the global biomarkers market in 2016, primarily due to the increasing use of reagent kits and assays for biomarker testing and the higher frequency of purchase of consumables. Although the price of consumables is lower than that of screening and automation instruments, consumables have a higher share in this market owing to their larger sales volume. As such, growth in the overall biomarkers market will be the most important driver for this product segment. Furthermore, the introduction of singleplex platforms, multiplex platforms, and automated ELISA systems that require assay kits is expected to aid the growth of this product segment.
Biologics Market:
The biologics showcase is significantly determined by developing capital venture from the key market players, the ascent in the pervasiveness of interminable sicknesses, loss of patent eliteness of the main biologic medications, and developing interest and higher worthiness for creative treatments The achievement of biologic medications in the treatment of major interminable sicknesses, quite malignant growth and immune system illnesses, has empowered enormous pharmaceutical organizations, as Eli Lilly and Company, Bristol-Myers Squibb, Novartis, AstraZeneca, and GlaxoSmithKline (GSK), to put resources into these items. In 2016, biologics represented six of the best eight medications, as far as income. Humira (adalimumab), which was created by AbbVie for the treatment of rheumatoid joint pain, psoriasis, Crohn's, and other immune system maladies, prompted USD 16 billion in deals, according to the report, Top 15 Humira was likewise the quickest developing natural medication. Amgen and Pfizer created Enbrel, which is utilized for treating polyarticular adolescent idiopathic joint inflammation in patients matured two years or more established and plaque psoriasis in patients matured four years or more Novartis AG put around USD 700 million in its biologics office in Singapore, and Bristol-Myers Squibb put almost USD 900 million out of an Irish huge scale biologics office that is foreseen to Moreover, the organizations are either procuring or converging with different organizations, for critical arrangements. For example, Shire obtained Baxalta to make a biologics-based item for the treatment of uncommon ailments and other particular issue. Pfizer purchased Medivation for the most part because of the prostate malignant growth tranquilize Xtandi. Pfizer additionally procured Anacor Pharmaceuticals to reinforce its aggravation and immunology pipeline.Thus, the developing extension and interest in biologics by significant organizations are relied upon to prompt the proceeding with blast in biologics.
Conference Highlights
- Biosimilars
- Biologics
- Emerging Biosimilars in Therapeutics
- Pharmacovigilance challenges in Biosimilars
- Biological Medicine
- Biopharmaceutical Informatics
- Chemical and Analytical Strategies for Biosimilars
- Bioavailability and Bioequivalence
- Biosimilars Development in Markets
- Immunogenicity of Biosimilars
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | November 26-27, 2021 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
Abstracts will be provided with Digital Object Identifier by


